Many patients find that certain over the counter and prescription eye drops do not help dry e ye or even make their dry eye symptoms worse. It is true that many OTC drops contain preservatives which can damage corneal and conjunctival cells if used over time (we do not have hard proof yet that this included meibomian gland cells but likely excessive preservatives damage MG cells also). Some patients are allergic to even mild preservatives in OTC and prescription drops.
ye or even make their dry eye symptoms worse. It is true that many OTC drops contain preservatives which can damage corneal and conjunctival cells if used over time (we do not have hard proof yet that this included meibomian gland cells but likely excessive preservatives damage MG cells also). Some patients are allergic to even mild preservatives in OTC and prescription drops.
The ingredient that causes this is Tetrahydrozoline which is a sympathomimetic amines. It works by temporarily narrowing the blood vessels in the eye. This narrowing occurs in all blood vessels as it does not discriminate between "ugly" blood vessels and normal vessels. The rebound redness occurs as a response of the body to this narrowing as well as the direct toxic effect of this chemical to the surface cells of the eye over chronic use of the drug. Avoid Tetrahydrozoline as much as possible.
Active Ingredients:Glycerin 0.2% (Lubricant)Hypromellose 0.2% (Lubricant)Polyethylene glycol 400 1% (Lubricant). Inactive ingredients:ascorbic acid, benzal
This is why n
Retaine remains the most loved non-preserved/preservative free eye drop among my patients.
Rarely does a patient not like Retaine but I have seen some patients who say it makes them feel worse or does not help.
But there are many artificial tear options.
Doctors and surgeon generally prefer to use natural remedies when proven. In dry eye, there are not many high volume-patient studies that are randomized, double blinded and controlled.
remedies when proven. In dry eye, there are not many high volume-patient studies that are randomized, double blinded and controlled.
We do know that the tear film's health is dependent on a good diet. What that diet should entail is ve ry controversial. It is well known that malnutrition, Vitamin A deficiencies, hyperlipidemia (high cholesterol) and diets low in Omega 3 negatively impact the tear film function and can lead to symptoms and signs of dry eye.
ry controversial. It is well known that malnutrition, Vitamin A deficiencies, hyperlipidemia (high cholesterol) and diets low in Omega 3 negatively impact the tear film function and can lead to symptoms and signs of dry eye.
I try, as much as possible, to slip in Omega 3 into our diet. I wi
We frequently take supplements, though.
In order of preference, the below Omega 3 supplements are the ones I, my family, and my colleagues use: PRN is the most expensive. It is the only one with a good publication to say it is the best but the company sponsored the study. Still, my husband swears this has helped his dry eyes better than any of the other brands I have bought, so we swallow the bitter pill of the cost to help him feel relief.
I prefer liquid versions of Omega 3.
I have also used these for the kids:
I have also tried these which I liked:
Dr. Martinez, my colleague loves UDO's Oil:
Many studies have investigated the effectiveness of oral supplementation with antioxidants, omega-3 (e.g. fish oil and linseed oil) and omega-6 (e.g. evening primrose oil) fatty acids in the last decade.At most, the studies are small and show a small benefit of oral supplementation on tear film volume, stability and decreased ocular symptoms in patients previously diagnosed with ocular surface diseases involving the ocular surface (e.g. Sjögren’s syndrome, meibomian gland dysfunction, dry eye disease) and contact lens wearers suffering from dry eye. More research is required to determine the exact composition, dosage and indications for their use and to fully characterize how these nutraceuticals modulate the tear film.
A published study in Contact Lens and Anterior Eye noted that the omega-6 fatty acids in evening primrose oil can help reduce dry eye symptoms in female contact lens wearers. After six months, the women in the trial who were administered evening primrose oil reported less eye dryness than those who received the placebo of olive oil.
In another study: patients suffering from dry eye symptoms who exhibited a chronic need of artificial tears were given
1. 2 capsules of 500 mg each of evening Primrose oil (Efamol-73% linoleic acid and 10% gamma-linolenic acid),
2. 50 mg vitamin B6 (pyroxidine) and
3. 1 g vitamin C
Three times a day.
Over 50% of those in the study showed substantial improvement within 2-6 weeks.
Again this is a small study & am looking for a better study to prove it's effects. I could not find 1 pill with all 3 items in one.
SLC:
The full study is below: **
In Brief:
Dry eye or Keroconjunctivitis Sicca and other surface disorders is usually a chronic condition. We have no cure. The remedies should focus on 2 aspects:
1. Saving all eye surface cells and meibomian gland cells
2. Palliative care: meaning treat the symptoms to avoid chronic inflammation which can show itself as chronic redness, irritation, burning.
, with the administration of lubricating drops. Current topical products are formulated to both lubricate the eye and enhance certain characteristics of the tear film. For example, hypotonic solutions reduce osmolarity and mucolytic agents can decrease the symptoms of excess mucin strands. Other additives may help lower tension at the water-oil interfaces and mimic some actions of the mucin network. However these palliative measures are temporary and do not address the underlying causes. For example, reduced osmolarity upon instillation may last only about 10 minutes.
Dry eye or Keroconjunctivitis Sicca and other surface disorders is usually a chronic condition. We have no cure. The remedies should focus on 2 aspects:
1. Saving all eye surface cells and meibomian gland cells
2. Palliative care: meaning treat the symptoms to avoid chronic inflammation which can show itself as chronic redness, irritation, burning.
, with the administration of lubricating drops. Current topical products are formulated to both lubricate the eye and enhance certain characteristics of the tear film. For example, hypotonic solutions reduce osmolarity and mucolytic agents can decrease the symptoms of excess mucin strands. Other additives may help lower tension at the water-oil interfaces and mimic some actions of the mucin network. However these palliative measures are temporary and do not address the underlying causes. For example, reduced osmolarity upon instillation may last only about 10 minutes.
A more effective approach may be to address the biochemical basis of an intact tear film. D.F. Horrobin and colleagues have carried out some preliminary studies of the use supplemental intake of essential fatty acids, vitamin B6, and vitamin C to treat dry eye. The rationale for this treatment was based on the biosynthesis of prostaglandin E1 (PGE1), which is necessary for aqueous tear secretion by the lacrimal glands.
- Linoleic Acid
- Gamma Linolenic Acid
- Di-hommo Gamma Linolenic Acid
- PGE1
- Di-hommo Gamma Linolenic Acid
- Gamma Linolenic Acid
Methods:
In this pilot study, 17 patients were selected based on failure of tear secretion, objectively demonstrated (Schirmer Test), clinical exam, and the chronic need/use of lubricant drops. Patients received 2 X 500 mg capsules of evening Primrose oil (Efamol- 73% linoleic acid and 10% gamma linolenic acid), 50 mg vitamin B6 (pyridoxine) and 1 g vitamin C three times daily
Results:
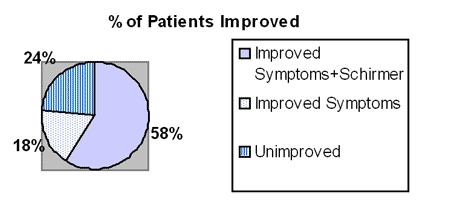
Ten of the 17 patients showed substantial improvement of both symptoms and Schirmer test in 2-6 weeks. 3 additional patients reported improved symptoms though without demonstrating improved Schirmer. The authors conclude that this treatment approach is effective in many cases. They continue to work on dose optimization and plan further testing.
**
TREATMENT OF THE SICCA
SYNDROME AND SJOGREN'S SYNDROME
WITH E.F.A., PYRIDOXINE AND VITAMIN C
D. F. HORROBIN, A. CAMPBELL, C. G. MCEWEN
P.O. Box 10 Nun's Island, Montreal H3E lJS, Canada I Hairmyres Hospital, East Kilbride 2
and Glasgow Eye Infirmary, 3 San@ford Place 3, Glasgow, Scotland
INTRODUCTION
In Sjogren's syndrome and the sicca syndrome, there is failure of normal secretion of
tears and saliva. ~ In Sjogren's syndrome, this failure is associated with a "connective
tissue" disease, often rheumatoid arthritis, while in the sicca syndrome the eye abnormalities
may occur either alone or coupled with loss of saliva. Although many therapies
have been tried, none is effective and the patients must resort to frequent use of lubricant
eye drops and lubricant mouth washes, Patients with either syndrome cannot wear
contact lenses and have difficulties watching television, eating and speaking.
A number of observations suggest that the failure of tear secretion may be related to
inadequate formation of prostaglandin (PG) E13: (1) Deficiency of essential fatty acids
causes lacrimal gland atrophy in animals. 1 (2) Deficiency of pyridoxine which seems
important in conversion of linoleic acid to dihomogammalinolenic acid (DGLA) causes
lacrimal gland atrophy. 7 (3) In humans, lack of vitamin C which seems important in
PGEx formation s caused features of Sjogren's syndrome to appear in 5/5 individuals who
went on a vitamin C deficient diet. 2 (4) The animal model of Sjogren's syndrome is the
NZB/W mouse. Many of the pathological features in these animals can be prevented by
PGE~ treatment. '~ (5) Of 8 PGs tested, only PGE1 had a significant effect in increasing
tear production in rabbits. 6 It, therefore, seemed worth attempting to treat the dry eye
syndrome with essential fatty acids, pyridoxine and vitamin C. One of us (AC) had
previously tried to treat the syndrome with high doses of vitamin C alone with no
SuCCeSS.
CLINICAL REPORTS
Patients were selected for the study on the basis of failure of tear secretion, objectively
demonstrated and necessitating frequent use of lubricant eye drops. Patients were
assessed by clinical observation, by objective measurement of the rate of tear production
using the Schirmer test, by subjective reporting and by changes in the use of lubricant eye
drops. Seventeen patients have been entered into the study to date. Each patient has
received 2 x 500 mg capsules of evening primrose oil (Efamol) containing 73% of linoleic
acid and 10% of ~-linolenic acid, 50mg of pyridoxine and 1 g of vitamin C 3 times per
day. In nine of the patients, the dry eyes were associated with some form of connective
tissue disease (Sjogren's syndrome) while the other 8 had various forms of the sicca
syndrome. In 3 of the 8, the sicca syndrome had been induced by use of the E-blocking
drug, practolol.
To date, 10 patients have shown both subjective and objective evidence of either
substantial improvement or complete resolution of the dry eye syndrome. One patient
who had been unable to undergo necessary ophthalmic surgery because of absence of
tears has been successfully operated upon. All 3 practolol patients were in this group of
10. The improvement took 2-6 weeks to become apparent and appeared to continue
progressively over several weeks. In 3 patients, although there was no increase of tear
production on Schirmer testing, the eyes appeared moist, the patients felt better and they
253
254 D.F. Horrobin, A. Campbell and C. G. McEwen
reported decreased use of eye drops. In 2 patients, there was objective evidence of
increased tear production but this was not accompanied by any subjective sense of
improvement. Two patients have shown no change after 2 months therapy. Two patients
who had suffered for many years from poor nails which broke frequently, reported a
sharp improvement in nail quality during the treatment.
CONCLUSIONS
In the majority of patients with the dry eye syndrome, the combination of evening
primrose oil, pyridoxine and vitamin C produced a substantial improvement. This is the
first report of a safe, systemic therapy for this disease. Some of the patients are still
improving. The syndrome is often associated with fibrosis and degeneration of the lacrimal
glands. It is possible that in some individuals this has progressed to an irreversible
stage while in others many months of treatment may be required before recovery.
To date, only one dose regime has been tested. It is possible that other doses of one or
other of the components of the regime may be more successful than reported here.
Further work is in progress to determine the optimum dose levels to be used. Once this
regime has been worked out, a placebo-controlled double blind trial will be set in
motion.
REFERENCES
I. AL^M, B. S. and ALAM, S. Q. Proc. Soc. Exp. Biol. Med. 161, 199-203 (1979).
2. Hoot), J., BURNS, C. A. and Hot)o~, R. E. New Enol. 3. Med. 282, 1120-1124 (1970).
3. HoaaoaiN, D. F. and C^MPaELL, A. Med. Hypothese~ 6, 225-232 (1980).
4. KRAK^UER, K., TOR~tEV, S. B. and ZURIER, R. B. Clin. Immunol. Immunopathol. II, 256-264 (1978).
5. MA~<u, M. S., OKA, M. an~ H~p,l~o9i~, D. F. Prostaglandins Med. 3, I19-126 (1979).
6. PHOI.PP, A~OOL, C. Prostaolandins Med. 3, 185-192 (1979).
7. SHI~.aN, M. A. Sjooren's Syndrome, W. B. Saunders, Philadelphia, 1971.
Ref:
https://www.sciencebasedhealth.com/Webpage.aspx?WebpageId=74
References & More notes:
Omega-6 fatty acids include linoleic (LA) and gamma-linoleic (GLA) acids which can metabolize into prostaglandin E1 (PGE1) – an anti-inflammatory mediator - or into arachidonic acid (AA), a precursor for the pro-inflammatory mediators prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) (James et al., 2000). As EPA and AA act competitively for the enzymes cyclooxygenase and 5-lipoxygenase, the modulation of inflammatory activity in the body is thought to be based on the balance of EPA/AA in the body (James et al., 2000). Synthesis of the longer chain omega-3 fatty acids within the body is competitively slowed by the omega-6 analogs. Thus, accumulation of long-chain omega-3 fatty acids in tissues is more effective when competing amounts of omega-6 analogs do not greatly exceed the amounts of omega-3. Omega-6 rich animal foods include meat, poultry and eggs whilst plant sources include evening primrose and borage (also called starflower) oils.
4.2. Omega-6 formulations
Ten trials have investigated formulations of omega-6 alone or combined with antioxidants (Aragona et al., 2005; Barabino et al., 2003; Horrobin et al., 1981; Kokke et al., 2008; Macri et al., 2003; Manthorpe et al., 1984; McKendry, 1982; Oxholm et al., 1986; Pinna et al., 2007; Theander et al., 2002). The first anecdotal report of effectiveness of omega-lipid-based dietary nutraceutical was published in 1981 (Horrobin et al., 1981). This trial reported “resolution” based on increased Schirmer results (i.e. tear volume) or decreased use of lubricating drops in 10 of 17 aqueous deficient dry eye patients (including Sjögren's syndrome) ingesting 1 g evening primrose oil and antioxidants (730 GLA, 100 mg DGLA, 1g vitamin C, 50 mg vitamin B) for a minimum of 8 weeks (Horrobin et al., 1981). No data was reported (Horrobin et al., 1981). A RCT investigating the effectiveness of two doses (800 mg GLA versus 1600 mg GLA) for six months in Sjögren's syndrome patients detected no differences in symptoms, tear volume or stability between the two groups (Theander et al., 2002). Equal bioavailability of GLA and DGLA in plasma of both groups at 3 months suggested that the corn oil used in formulating the placebo may be a confounder (Theander et al., 2002). Similarly, a trial investigating the effectiveness of a large dose of 3 g evening primrose oil with vitamin C (2190 mg GLA, 300 mg DLGA, 3 g vitamin C) in patients with Sjögren's syndrome for 10 weeks demonstrated no changes in symptoms or tear volume (McKendry, 1982). The sample size of 10 subjects used may have been too small to show an effect and use of systemic non steroidal anti-inflammatory medication by six of 10 patients is a potential confounder (McKendry, 1982).
Conversely, a RCT investigating a combination of evening primrose oil and antioxidant (240 mg GLA, 81.6IU vitamin E, 750 mg vitamin C, 150 mg pyridoxine, 150 mg niacin, 30 mg zinc) in Sjögren's syndrome patients for three weeks demonstrated improved tear volume but no effect on tear stability, ocular surface staining, corneal sensitivity, symptoms, conjunctival morphology or levels of tear lysozyme (Manthorpe et al., 1984). It is possible that the study period may have been too short to demonstrate effectiveness (Manthorpe et al., 1984). A second RCT investigating the evening primrose oil (240 mg GLA) without antioxidant in 28 Sjögren's syndrome patients for eight weeks demonstrated less ocular surface staining and better overall combined score of tear volume, tear stability and ocular surface staining (Oxholm et al., 1986). Individual differences in tear volume and tear stability or levels of tear lysozyme could not be demonstrated (Oxholm et al., 1986). A third RCT more recently demonstrated improved symptoms, reduced corneal staining and increased levels of PGE1 in the tear film of Sjögren's syndrome patients ingesting 225 mg LA and 30 mg GL daily for 1 month but no effect on tear stability or tear volume (Aragona et al., 2005).
Two studies used low supplementation with 57 mg LA and 30 mg GLA (Barabino et al., 2003; Pinna et al., 2007). The first trialled this for 45 days in a RCT in subjects diagnosed with aqueous deficient dry eye and demonstrated reduced conjunctival expression of the inflammatory marker HLA-DR and improved symptoms and ocular surface staining, however this was not accompanied by any changes in tear stability or tear volume (Barabino et al., 2003). The second study compared the effectiveness of the same supplement in subjects diagnosed with meibomian gland dysfunction against lid hygiene treatment over 180 days. Improvements in symptoms and signs of meibomian gland dysfunction occurred in both groups with possibly larger improvements in the group receiving dietary supplementation (Pinna et al., 2007). The trial unfortunately did not include a placebo control group and is subject to significant bias; it also did not include any measure of the tear film characteristics (Pinna et al., 2007).
Finally, a RCT investigating the effectiveness of evening primrose oil (300 mg GLA) for contact lens dryness over 6-month demonstrated improved comfort and tear volume but no effect on tear stability, ocular surface staining or meibomian gland appearance (Kokke et al., 2008). An investigator masked no-treatment control trial investigated the effectiveness of using low supplementation with 28.5 mg LA and 15 mg GLA for 1 month in patients undergoing photorefractive keratectomy (PRK) to correct their refractive error (Macri et al., 2003). Participants ingesting omega-6 nutraceuticals had improved tear volume and tear clearance and reported better comfort but no differences in ocular surface staining were detected (Macri et al., 2003).
A 6-month uncontrolled open-label trial of a combination omega-3 and -6 (1000 mg linseed and 500 mg evening primrose oil) suggested improvement in symptoms and tear stability; the results are unfortunately subject to significant bias as neither the investigator nor the patients were masked and no placebo control was used (Jackson et al., 2011). A 3 month RCT involving a sea buckthorn oil from the sea buckthorn berry (245 mg LA, 149 mg ALA, 346 mg palmitoleic acid (omega-7), 316 mg oleic acid, 108 mg cis-vaccenic acid (omega-7), 6.8 mg vitamin E, 1.8 β-carotene & zeaxanthin) was recently conducted in a cohort of 100 Finnish patients. Although there were no differences between the two groups, there was less increase in tear osmolarity and reduced symptoms in the test than the control group (Larmo et al., 2010). This indicates that sea buckthorn oil treatment minimized the worsening of the clinical signs and symptoms rather than improved them.













No comments:
Post a Comment
Note: Only a member of this blog may post a comment.